Facility Focus: Advantages of Titanium in Corrosion Resistance
One of the key concerns expressed by Athletic Directors when discussing the future of college swimming and diving is the facility lifecycle. As facilities age, the cost of maintaining them increases. These costs factor in to the strategic decisions of athletic departments meaning it is important for coaches to consider ways to increase the lifecycle of their facility. Here's one such way:
ABSTRACT 1, 2
Titanium, a metal well known for its strength, light weight and durability, possesses qualities that grant it high resistance against the corrosive nature of aquatic environments. Colorado Time Systems did extensive research and conducted experiments analyzing these qualities. It has been shown that titanium’s ability to withstand corrosion makes it one of the best metals for manufacturing certain components of aquatic equipment; a practical application being deck plates.
INTRODUCTION 3, 4, 5, 6
When designing products that are going to be used in the pool environment, it is important to anticipate corrosion because corrosion can be detrimental to swim timing equipment. When it builds up to appreciable levels, the timing system won’t work. Some corrosive threats include water sterilization chemicals, humidity, and water itself.
Pool disinfectants such as chlorine, bromine, and salt are highly corrosive. These chemicals can also produce corrosive byproducts if the pH level of the pool water goes below 7.4. For example, insufficient free chlorine can lead to chloramine compounds when it breaks down biological material.
Chloramines are the source for the “pool smell” upon evaporation, and have intense corrosive properties in the warm indoor pool environment. Chloramines evaporate into warm air and then settle on cooler surfaces as condensation. When chloramines condense they break down, corroding and attacking underlying surfaces. This puts pool components outside the water at risk.
MATERIAL SELECTION 5, 7, 8
Because the pool environment is harshly corrosive, the type of metal used to manufacture pool and swim timing equipment must be chosen carefully in order to ensure the longevity of its use.
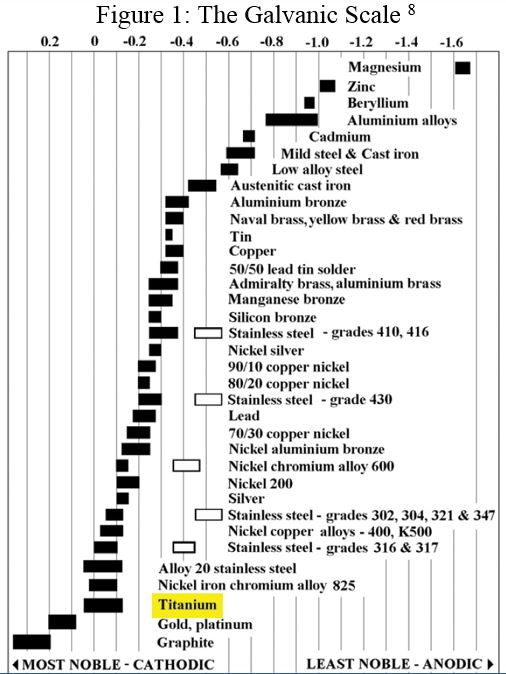
On the Galvanic Scale titanium is only outranked by metals such as gold, platinum, and graphite, making it noble and highly corrosion-resistant. Certain characteristics of the outranking metals, such as cost and low mechanical stability, eliminate them as viable prospects. Titanium has the advantages of being more cost effective, light-weight, and as strong as steel.
High performance metal alloys, such as Hastelloy and Inconel, also have excellent mechanical strength and are corrosion-resistant. However, they are more than twice as expensive as titanium.
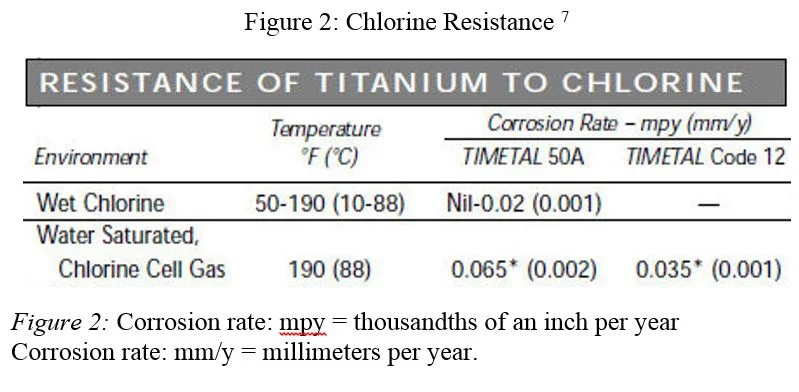
As predicted by titanium’s place on the Galvanic Scale, its resistance to corrosion by chlorine is extremely high. The table below describes titanium’s resistance to high concentrations of chlorine in hot conditions. In this accelerated climate (far worse than any swimming pool) it would take approximately 1,900 years for titanium to erode an eighth of an inch.
CHEMISTRY 7, 9, 10
Another valuable attribute of titanium is its capacity to form a shield against corrosive agents, guarding itself from deterioration.
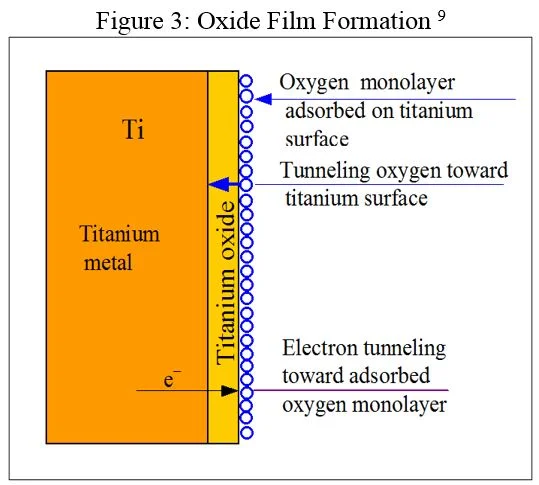
Oxygen and water together cause oxidation of many materials, resulting in corrosion. When titanium comes in contact with oxygen and water, instead of corroding, it produces an oxide film that is invisible to the naked eye. The film’s thickness is typically a hundredth of a micron, or roughly 1/10,000th the width of a human hair. This film coats and protects the surface of the titanium and continuously gets stronger over time, constantly enhancing resilience against corrosive agents.
Few substances can breach or damage this protective film, and if the oxide film is mechanically fractured it regenerates immediately. This feature, called passivation, vastly lengthens the continued usability of titanium and is involved in its high valuation on the Galvanic Scale.
PRACTICAL APPLICATION 1, 2
Certain aquatic components, such as deck plates for competitive swimming, can be more prone to accelerated corrosion. Deck plates can experience increased damage from corrosive agents due to their location and function.
Deck plates are installed at the end of each swim lane and send and receive data to and from the timing system for the swimmer in that lane. Because of their location close to the pool they are frequently splashed with pool water. As a deck plate experiences alternating encounters with pool water and air, a cycle of wet and dry is established. This cycle is even more corrosive than complete immersion in pool water as the concentrations of pool chemicals increase when pool water dries up and is replenished repeatedly.
In addition, when the timing system is connected to the deck plate a very low electrical voltage is introduced into the system to detect the timing signals. Pool water contains ions that make it slightly conducting, and a corrosive reaction called electrolysis occurs when pool water creates a bridge between the electrical connectors. The signal voltage for the connected devices brings forth an electrolytic current through the water bridge between the connectors.
While there is no danger of shock, this current facilitates the transportation of ionic material and leads to accelerated corrosion, requiring high maintenance for traditional deck plates in terms of cleaning and replacement. This corrosion causes contact resistance to increase dramatically, which means eventually signals cannot get through to the timer.
To alleviate the effect of these corrosive conditions, Colorado Time Systems has patented a new deck plate design using what is called domed topography. The domed topography allows water to easily run off the deck plate after a splash, helping to tremendously shorten the wet part of the wet and dry cycle and preventing water from collecting around the connectors or between their contacts.
Electrolysis occurs even when a deck plate is briefly splashed, so Colorado Time Systems also uses titanium connectors for the new deck plate design. Since titanium is inert and protected by its layer of titanium oxide, it is more impervious against damage from electrolysis.
RESEARCH
When preparing the new deck plate design, a research and development team at Colorado Time Systems conducted an investigation regarding corrosion resistant materials. After narrowing the field and selecting titanium as the most viable option, the team then determined its levels of corrosion resistance and environmental eligibility. This was done by testing it in conditions of exaggerated severity compared to the pool environment. The traditional metal alloys currently used for deck plates underwent the same tests.
Titanium deck plate connectors and traditional deck plate connectors (composed of nickel-plated steel with conductors of a nickel-plated copper alloy) were exposed to wet chlorine concentrations about 10 times higher than that of regular pool water. After six months of this exposure the titanium connectors experienced no corrosion and worked properly. In contrast, after only a few days the traditional connectors sustained severe corrosive damage and could no longer function.
In addition to examining chlorine corrosion resistance, defense against muriatic acid was observed as well. Muriatic acid, a diluted form of hydrochloric acid, is also very corrosive. It can be used to lower the pH of pool water, and a diluted solution of about 2% is used to clean outdoor pool decks.
The muriatic acid used for this experiment was not diluted, and contained about 25% hydrochloric acid. The titanium connectors were exposed for three months. Although they were less shiny and grey after this exposure, the titanium connectors still worked properly for the timing signals and exhibited no degradation to the electrical connectivity caused by corrosion.
CONCLUSION
The investigation Colorado Time Systems conducted demonstrated titanium as a strong corrosion-resistant metal in the pool environment. Because of the inertness of titanium and the oxide film titanium generates to protect itself, it is clear that the use of titanium can improve the durability of aquatic equipment. It is critical to manufacture aquatic equipment capable of withstanding the corrosive pool environment.
As a result, installing deck plates that are manufactured using titanium will save facilities both time and money. This innovation will ensure product reliability when in use, decrease and even prevent unanticipated replacement/repair costs, and establish higher standards for aquatic equipment.
In response to the needs of the aquatic community, Colorado Time Systems is currently the only manufacturer of swim timing equipment that uses titanium as its metal of choice for various connections close to the pool water. This advancement, along with the patented domed topography, can immensely extend the operational usage of deck plates. Progress and further development will yield additional innovation in the future and raise standards for aquatic equipment.
Reference List:
- John Jr., M. A. (2002). Titanium- Properties, Advantages, and Applications Solving the Corrosion Problems in Marine Service. Denver: NACE International.
- Stockinger, C., Ryerson, C., & Anderson, B. (2014). Patent No. 8602815. United States.
- Pahlén AB. (2015). pH and Chlorine Values for Good Water Quality. Retrieved July 30, 2015, from Pahlén P: http://www.pahlen.com/users-guide/ph-and-chlorine
- Schechter, D. (2007). Myths, Maintenance, & Problems about Salt Water Pools. Dallas, Texas, United States.
- Nickel Development Institute; Sports Council; Building Research Establishment; Stainless Steel Advisory Centre; Institute of Sport & Recreation Management; Pool Water Treatment Advisory Group;. (2004). Stainless Steel in Swimming Pool Buildings. West Midlands: Nickel Institute.
- Griffiths, T. (2005, August). Clearing the Air: Chloramine Control for Indoor Swimming Pools. Retrieved July 2015, from Water and Health: http://www.waterandhealth.org/newsletter/cleaning_air.html
- Timet. (1997). Corrosion Resistance of Titanium. Henderson: Titanium Metals Corporation.
- Atlas Steels. (2010). Atlas Tech Note No. 7; Galvanic Corrosion. Australia: Atlas Steels.
- Tadeusz, H., Rokicki, R., & Rokosz, K. (2011). Magnetoelectropolished Titanium Biomaterial. In R. Pignatello, Biomaterials Science and Engineering (pp. 228-229). Croatia: InTech.
- Donachie, M. J. (2000). Corrosion Resistance. In M. J. Donachie, Titanium: A Technical Guide, 2nd Edition (pp. 125-126). Novelty: ASM International.
About Colorado Time Systems: Colorado Time Systems is an American company based in Loveland, Colorado that designs, manufactures, sells, and services aquatic timing systems, scoreboards, LED video displays, and related products.